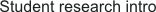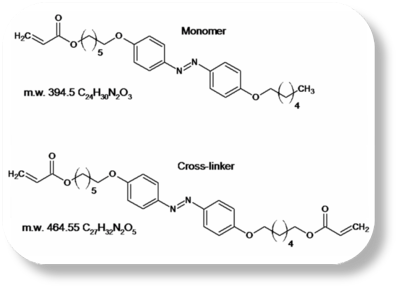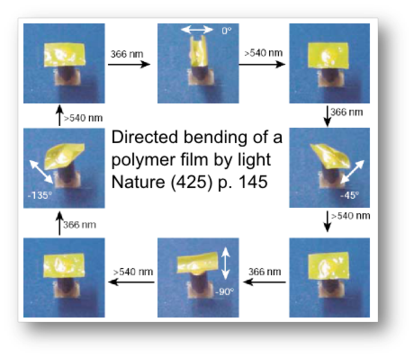
This LCE material is based upon an azo-benzene [benzene-N=N-benzene] mesogen. The azo-benzene group undergoes a reversible cis-trans conformational isomerization when exposed to light of the appropriate wavelength. The absorption of light produces results in a bending of the molecules that decrease the distance between first carbon atom of the benzene ring from 9 to 5.5 angstroms. Capitalizing on this isomerization process, LCE that contain azobenzene moieties may undergo significant change in dimension when exposed to UV light, as shown in the picture to the left. Prof. Ikeda's work using this material have resulted in a light driven plastic motor, and has been feature on NHK and several Japanese newspapers as well as leading research journals..
This material is prepared in a rubbed polyimide glass cell with the final LCE polymer network prepared photo-polymerization in the cell at the appropriate temperature. Material preparation involves the following steps.
- Chemical synthesis
- Mixture preparation (dark room)
- Cell preparation
- Cell loading & photo-cross-linking
- Release from cell & optical characterization