Liquid crystal elastomers (LCE) are a hybrid material that combine
liquid crystal orientational order with the elastic properties of a polymer network component into a single
composite material. Quoting Mark Warner's tome entitled appropriately enough, 'Liquid Crystal Elastomers', 'LCE are bring together, as nowhere else, three important ideas: orientational
order in amorphous soft materials, responsive molecular shape and quenched topological constraints.'
LCE materials are comprised of a liquid crystalline linear polymer with a low density of crosslinks.
This cross-linking differentiates the LCE from a simple LC polymer.
The resultant material has a unique coupling between anisotropic order of the LC component and elasticity of the polymer network,
given by the following equation:
It is beyond the scope of this website to describe the physics of LCE materials, and in addition to the literature,
we enthusiastically recommend Mark Warner & Eugene Teretnjev's book 'Liquid Crystal Elastomers'.
This coupling results in materials that exhibit unique properties in response to stimuli, such as dramatic
reversible change of dimension of more than 400%. To put this in perspective,
ideally, this corresponds to a one meter
piece of material reversible contracting to 12.5cm on the order of milliseconds.
Nobel laureate Pierre deGennes provided the first theoretical description of LCE in 1969,
with the first monodomain LCE films prepared in Heino Finkelmann's lab in the early 1990s.
Since then, a wide variety of LCE materials have been developed by researchers around the globe.
We begin our discussion of LCE with a brief discussion of the chemistry involved in preparing LCE materials
LCE are composed of a minimum of three components:
Preparation of an LCE involves covalently linking three components in some manner, we begin our discussion
with methods for creating a polymer network, followed by a brief discussion of mesogens structure, connectivity, and material
response.
The most commonly used techniques for making connections between the polymer and
mesogen & cross-linker
are the hydrosilylation reaction or acrylate polymerization, briefly desribed below. In both cases, the mesogen and crosslinker molecules are functionalized;
vinyl group for the hydrosilylation reaction, acrylate group for the acrylate polymerization. For these materials, statistically
random polymers are formed, meaning there is no predetermined, specified arrangement of the mesogen and the crosslinker.
Using either reaction method, nematic, smectic, cholesteric LCE can be prepared.
Alternative methods for preparing LCE materials, such as using micro-phase separation to form effective cross-links
or using biopolymers also exist, and are discussed elsewhere in the site.
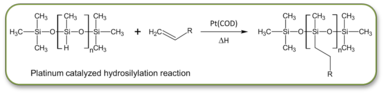
Methyl-hydrosiloxane polymers provide a highly reactive, inexpensive polymer network for making both main chain and side chain LCE films. The hydrosilylation reaction covalently links the LC mesogen or crosslinker with the siloxane polymer via a platinum catalyzed reaction. The siloxane polymer's Si-H group reacts with the a terminal double bond (vinyl or olefin) of the molecule you wish to attach to the siloxane polymer. The reaction is catalyzed by a platinum catalyst and can be run to completion at 40 to 60oC
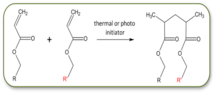
Polymerization of acrylate functionalized LC mesogens & cross-linkers provide another reaction scheme to make LCE films. The polymerization process is used initiated using a photosensitive or temperature dependent free radical initiator. The photo-initiator is more commonly used as it allows the reaction to be run at different temperatures, and corresponding different LC phases. For more information on acrylate LC polymers, please refer to the following: 'Photoinitiated polymerization and cross-linking of liquid crystalline systems' DJ Broer. Chapter 12, from the book Radiation Curing in Polymer Science, by Jean-Pierre Fouassier (Ed.), J.F. Rabek (Ed), 1993