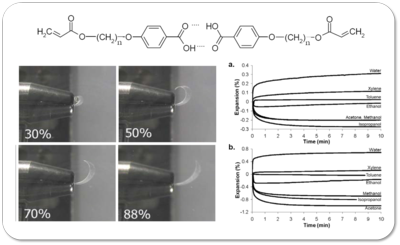
This side chain LCE has a unique mesogen structure that makes it responsive to chemical vapors. Unlike other LCE, the
mesogen of these materials contain hydrogen bonds to form the mesogens. The chemical structure of the hydrogen bonded
mesogen is shown at the top of the left hand image. When doped with either potassium or sodium ions,
these materials swell or contract with exposed to solvent vapor. The pictures at the bottom left show changes in the
structure of the LCE film when exposed to increasing relative humidity. As the
humidity increases and the film takes
up water vapor, it begins to expand along both axes, and uncurls. The bottom right plots change in film dimension
with exposure to different solvents for the long and short axis of the LCE film.
(ref. Self-Assembled Polymer Films for Controlled Agent-Driven Motion.
Nanometers (5)
, 2005
Thanks to Casper van Oosten of Prof. Broer's lab for his instruction on preparation of this material
This material is prepared in a cell coated with a poly-imide alignment layer. Nematic, smectic, splay and twisted nematic films may be prepared. The polymer network is formed by photo-crosslinking the material in the cell. After polymerization, the cell is opened and the material released from the surface.
- Chemical synthesis
- Mixture preparation
- Cell preparation & loading
- Release from cell
- Characterization
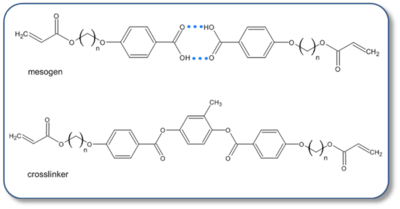
Chemical structure of the mesogen (top) and crosslinker (bottom). Individually,
the components do not adopt a LC phase. Hydrogen bonds, indicated as blue dotted lines between the individual components result in
a rod shaped LC mesogen similar to those found in other LCE. The number of carbons atoms in the flexible spacer
are 3, 5, & 6 carbon atoms.
Terminal acrylate groups are polymerized to form the LCE polymer
network.