 The gold nanorod (Au NR) synthesis procedures described on the website are a wet or 'green' synthesis procedure
developed by Nikoobakht and El-Sayed at Georgia Tech Univeristy. Using this procedure, water soluble
rods with aspect ratios, the ratio of length to width, ranging from approximately 2 to 5 can be easily prepared.
The completed Au NR have a surfactant coating of (Cetyl trimethylammonium bromide) CTAB. This procedure does
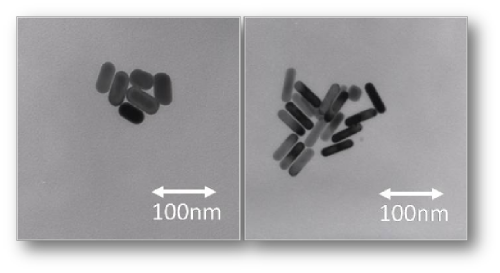
not require the synthesis of any starting materials. The images to the left
show Au NR of two different lengths, with the length controlled by altering
the amount of silver during the synthesis procedure. Included in the procedure is a method to coat the AuNR
with a polystyrene coating, making them soluble in organic solvents.
The gold nanorod (Au NR) synthesis procedures described on the website are a wet or 'green' synthesis procedure
developed by Nikoobakht and El-Sayed at Georgia Tech Univeristy. Using this procedure, water soluble
rods with aspect ratios, the ratio of length to width, ranging from approximately 2 to 5 can be easily prepared.
The completed Au NR have a surfactant coating of (Cetyl trimethylammonium bromide) CTAB. This procedure does
not require the synthesis of any starting materials. The images to the left
show Au NR of two different lengths, with the length controlled by altering
the amount of silver during the synthesis procedure. Included in the procedure is a method to coat the AuNR
with a polystyrene coating, making them soluble in organic solvents.
Synthesis is Au NR is readily scalable, and requires approximately 4 hours to complete, depending on the speed of the centrifuge. Material preparation involves the following steps. Making organic solvent soluble Au NR requires and additional 2 to 4 hours
- Measuring out chemicals
- Preparation of seed and mother solutions
- Coating for organic solvent soluble Au NR
- Centrifugation
Reaction progress can be monitored by UV-VIS spectroscopy, with the actual size and morphology of the Au NR checked by Transmission Electron Microscopy (TEM).
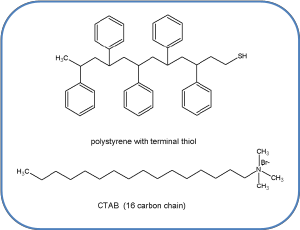
The Au NR are comprised of elemental Au atoms. Two types of coatings are commonly used, a non-covalent surfactant coating, in this case CTAB (shown above), or a covalently bound polymer coating such as polystyrene forming what is known as a brush to prevent aggregation of the nanoparticles. Thiol compounds, (molecules containing SH groups) covalently bind to the surface of Au NR.